1 Brazilian Health Surveillance Agency and Ministry of Agriculture WORKSHOP ON LABORATORY QUALITY SYSTEMS São Paulo, September 2003 Good Laboratory. - ppt download
MALAYSIA AS NON-OECD MEMBER ADHERING TO MUTUAL ACCEPTANCE OF DATA SYSTEM FOR GOOD LABORATORY PRACTICE

PDF) Developing laboratory capacity for Good Laboratory Practice certification: lessons from a Tanzanian insecticide testing facility
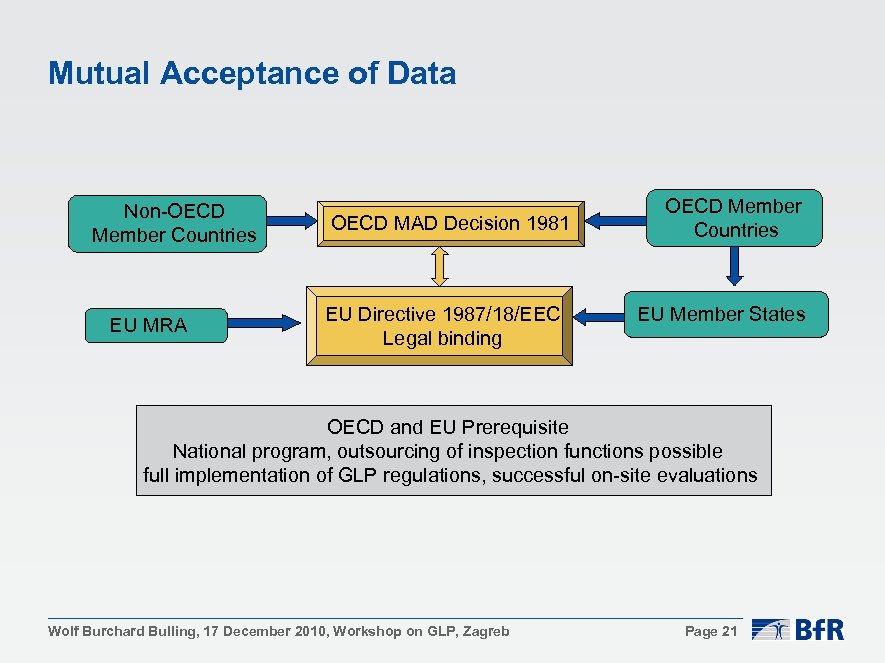
OECD Mutual Acceptance of Data System Factsheet What is OECD Mutual Acceptance of Data (MAD) system? 1. MAD was established in 1

OECD SERIES ON PRINCIPLES OF GOOD LABORATORY PRACTICE AND COMPLIANCE MONITORING Number 1 - PDF Free Download

FEDERAL INSTITUTE FOR RISK ASSESSMENT Good Laboratory Practice GLP in practice Dr. Wolf Burchard Bulling Federal Institute for Risk Assessment Thielallee. - ppt download

1 Brazilian Health Surveillance Agency and Ministry of Agriculture WORKSHOP ON LABORATORY QUALITY SYSTEMS São Paulo, September 2003 Good Laboratory. - ppt download
General Questions and Answers Concerning OECD Principles of Good Laboratory Practice (GLP) and Mutual Acceptance of Data (MA
Official Journal of the European Communities 9. 10. 1999 L 263/10 AGREEMENT on mutual recognition of OECD principles of good lab